Technical Cooperation
for Research at Chiba University
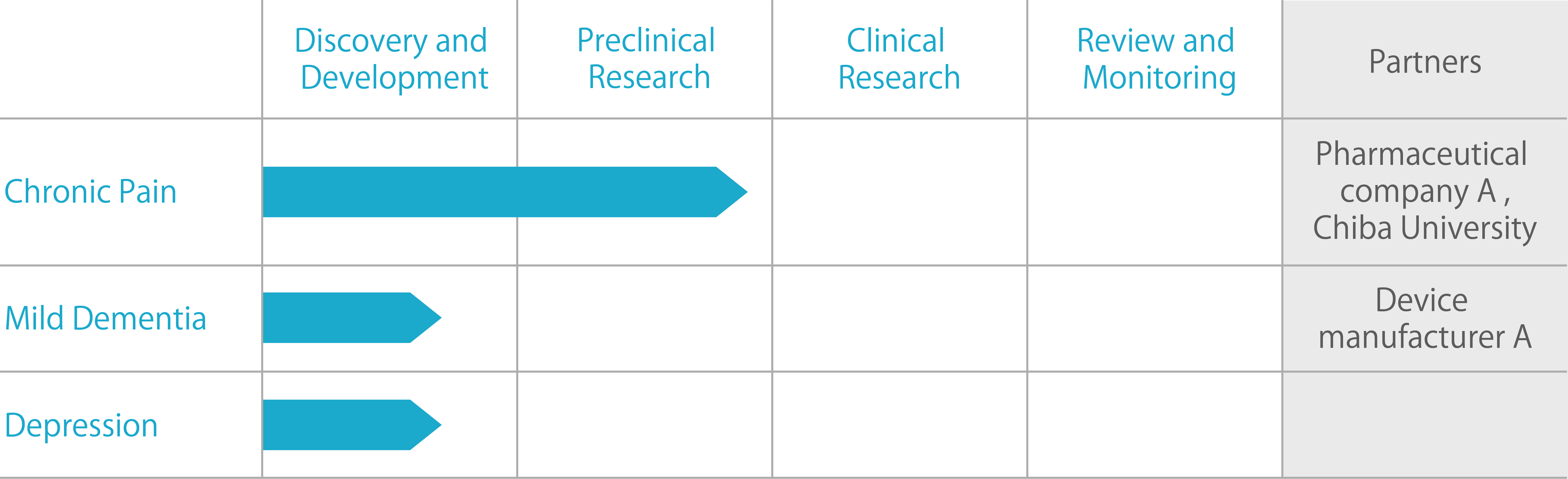
Hospital Pain Center

With an estimated economic loss of up to three trillion yen, back pain is said to be a national disease in Japan, and according to an announcement by the Ministry of Health, Labor, and Welfare, almost all (about 85%) patients suffering from back pain who visit hospitals as outpatients have non-specific back pain for which the reason cannot be identified.
At Chiba University Hospital's pain center, to conduct research measuring the effectiveness of various treatments on non-specific chronic back pain sufferers, along with cognitive behavioral therapy and exercise therapy, a new type of treatment making use of neurofeedback was verified.
For this neurofeedback, "ALPHA SWITCH", a neurofeedback engine uniquely developed and provided by MEDIASEEK as well as a smartphone app equipped with that engine were adopted, and it turned out that neurofeedback plays the role of increasing the effectiveness of existing treatments such as cognitive behavioral therapy. The research paper has been made public in "Scientific Reports", an international journal.

Mild Dementia
Screening Project
Dementia is one of the biggest social issues in Japan. The number of patients with dementia is expected to increase, with the Cabinet Office estimating that by 2030 there will be more than 7 million dementia patients in the country.
Since no cure for the root cause of dementia has yet been established, it is important to detect mild dementia, with mild symptoms, at an early stage.
For this early detection, MEDIASEEK has partnered with a manufacturer developing devices to research and develop a service that screens for mild dementia based on brain responses using BrainTech technology.